Research
Computational models to predict disease progression and phase transitions from multimodal data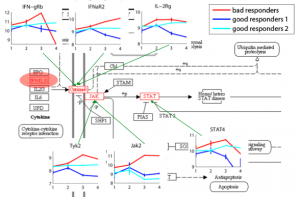 Gene expression and DNA methylation profiling allows clinical diagnosis to be made on a molecular level, thereby substantially increasing diagnosis accuracy and facilitating choice of treatment based on the patients’ genetic traits. Moreover, identifying disease-related genes and monitoring their activity levels provide insights into disease mechanisms. We aim to improve methods for disease diagnosis and marker selection by integrating additional sources of data and the design of methods capable of handling high dimensional spaces. |
Infering gene regulatory mechanisms driving cell differentiation and malignant transformations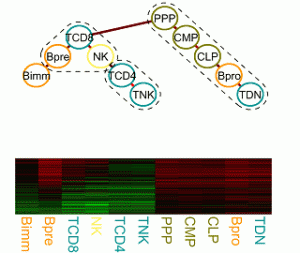
The regulatory processes that govern cell proliferation and differentiation are central to developmental biology. Particularly well studied in this respect is the hematopoietic system. Gene expression, regulation and epigenetic data of cells of various distinguishable developmental stages foster the elucidation of the underlying molecular processes, which change gradually over time and lock cells in certain lineages or induce cell potency. We are interested in developing a statistical framework for tasks as finding modules of functionally related genes and characterizing cell potency and commitment from expression and epigenetic signatures. |
Detection of Regulatory and Epigenetic Traits from Bulk and Single Cell Sequencing Data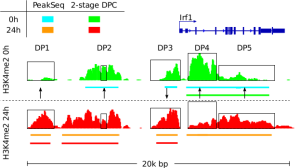 We develop methods for detection of epigenetic features of cell, such as cell active binding sites from open chromatin essays and chromatin changes during biological processes down to a single cell level. These methods include the integrative analysis of ATAC-seq, DNase-, ChIP-, and RNA-Seq and single cell variants of these protocols. In this context, we develop a toolbox to perform several basic computation tasks required for the analysis of regulatory genomics data: www.regulatory-genomics.org . |
